Introduction
- Water plays many essential roles in biological systems, including structural roles when bound within a biopolymer.
- Buried water takes part in substrate recognition in carbohydrate-binding enzymes, in protein kinase A, in amionacyl-tRNA synthetase, and in lipid binding proteins. Buried water is believed to play an important role in control of the specific conformation of HIV-1 protease for the initiation of enzyme reaction, and in the structure and function of aspartic proteinases, MHC class-I proteins, and the phospholipase OMPLA.
- Water plays a vital role in the molecular recognition between biological host and guest, and as such, it could prove useful in drug design to engineer water-binding sites into the interface between a drug and its biological target. However, studies which systematically probe and quantify these types of solvent-recognition effects with model receptors are lacking.
- This study is a component part of our project whose goal is the preparation of novel antibiotics which mimic the action of cationic peptides. Part of the innate immune system of such diverse organisms as plants, invertebrates, and vertebrates, these antimicrobial peptides exhibit a broad spectrum of activity against Gram-positive and Gram-negative bacteria by binding to and disrupting bacterial anionic cell wall components. Since these anionic wall groups are highly hydrated, the development of receptor-design strategies that include water molecules as part of the binding motif may prove to be of fundamental importance.
Progress To Date
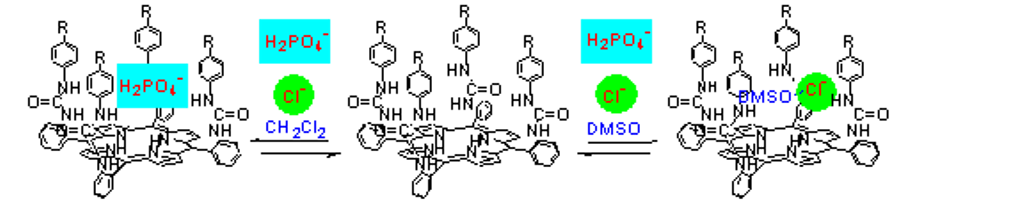
- Tetra-urea picket porphyrins will bind to a DMSO molecule and utilize it as a participant in its anion recognition unit, in a similar manner to enzymes that bind water for use as part of their substrate recognition unit.
- The receptor-bound solvent molecule determines the anion binding selectivity, affinity, and stoichiometry of binding. With a bound DMSO molecule, the receptor is highly selective for chloride anion. Absent the buried solvent molecule, the receptor binds more strongly to phosphate or nitrate anions. Indeed, the Keq for chloride anion in the non-competitive solvent CH2Cl2 is smaller than in the highly competitive solvent DMSO!
- A remarkable reversal in the selectivity of anion complexation between various picket porphyrin receptors is observed, wherein the binding constant ratios change over 3 orders of magnitude as the receptor's number of urea pickets change from four to two (porphyrins 1, 2b, 3b). The latter receptor has no urea pickets available to bind to solvent after complexation with an anion.
Summary
- Anion complexation with hydrogen bonding receptors in a competitive solvent is enhanced when a ubiquitous solvent molecule is incorporated into the binding motif, i.e., competitive solvent can add to the overall complexation energy and thereby strengthen binding via hydrogen bonding rather than weaken it, as commonly believed.
- Solvent incorporated into the binding motif of a (bisubstrate) receptor can determine its selectivity of anion binding.
- In other words, a receptor's binding pocket that is complementary for one anion can be modified and made complementary for a different anion with the binding and appropriate positioning of a solvent molecule.
- The data suggests that some drugs need not be completely desolvated to selectively bind to their biological target if water can be included in the binding motif.
- We're currently examining other types of receptors in order to develop parameters for the complexation of ubiquitous solvent to ligands and receptors.

