
Professor of Chemistry
Inorganic Chemistry
A.B., Harvard University, 1986
Ph.D., University of California at Berkeley, 1992
Post doctorate, Northwestern University, 1992-1996
Tel: 316.978.3238
Fax: 316.978.3431
Email: David.Eichhorn@wichita.edu
Research Interests
-
Metalloenzyme Model Complexes
-
Polypyrazolylborate Complexes
-
Conductive and Magnetic Coordination Polymers
Research in the Eichhorn group is in the area of synthetic inorganic chemistry. Two current projects, described below, are (i) the synthesis of coordination polymers using a new class of polypyrazolylborate ligands with cyano substituents and (ii) modeling the active sites of sulfur-coordinated metalloenzymes. The research effort involves synthesis of ligands and metal complexes, characterization of the metal complexes by a variety of spectroscopic and other analytical techniques, and structural analysis of the products by X-ray crystallography.
Cyano-substituted Polypyrazolylborates
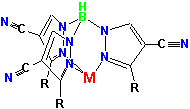
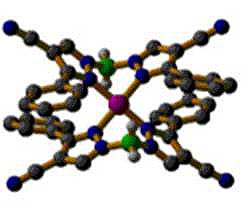
A new class of polypyrazolylborate ligands with cyano substituents (left) has been developed and is being studied as precursors to magnetic and conductive polymers. Right is shown the crystal structure of the copper complex of a cyano-substituted bispyrazolylborate ligand, Cu(BpPh,4-CN)2.
 |
 |
Thiolate-substituted Metal Complexes
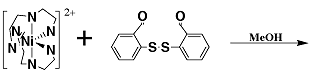
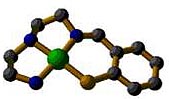
A new method for synthesizing metal complexes with mixed N/S coordination has been developed which uses an air-stable disulfide reactant. For example, the first Ni(II) complex of a tetradentate N3S donor ligand was synthesized by this technique. Compounds are being studied as models for the active sites of thiolate-coordinated metalloenzymes.
 |
 |
Selected Publications (Click for full publication list)
-
Zhao, N.; Van Stipdonk, M.J.; Eichhorn, D.M. “Sandwich Compounds of Cyanotrispyrazolylborates – Complexation-induced Ligand Isomerization”; Inorg. Chem. 2007, 46, 8662-8667.
-
Smucker, B.W.; Van Stipdonk, M.J.; Eichhorn, D.M. “Incorporation of Thiolate Donation Using 2,2'-Dithiodibenzaldehyde: Complexes of a Pentadentate N2S3 Ligand With Relevance to the Active Site of Co Nitrile Hydratase”; J. Inorg. Biochem., 2007, 101, 1537-1542.
-
Zhao, N.; Van Stipdonk, M.J.; Eichhorn, D.M. “Syntheses and Crystal Structures of 3-tert-butyl-4-cyano Pyrazole and its Complexes with Cobalt(II), Manganese(II), and Copper(II)”; Polyhedron, 2007, 26, 2449-2454.
-
Siemer, C. J.; VanStipdonk, M. J.; Kahol, P. K.; Eichhorn, D. M.; "A Coordination Polymer from a Cyanoscorpionate Complex"; Polyhedron 2004, 23, 235-238.
-
Eichhorn, D. M.; Goswami, N.; "New Synthetic Techniques for the Incorporation of Thiolate Donation in Transition Metal Complexes"; Comm. Inorg. Chem. 2003, 24, 1-13.
- Siemer, C. J.; Goswami, N.; VanStipdonk, M. J.; Eichhorn, D. M.; "Dihydrobis(4-cyano-3-phenylpyrazol-1-yl)borate: Homoleptic Mononuclear Cobalt(II) and Copper(II) Complexes With a Cyano-substituted Scorpionate Ligand."; Inorg. Chem., 2001, 40, 4081-4084.
-
Siemer, C. J.; Meece, F. A.; Armstrong, W. H.; Eichhorn, D. M.; "Iron, copper, and Cobalt Complexes of Hydrotris(3-phenylpyrazolyl)borate and Dihydrobis(3-phenylpyrazolyl)borate. Synthesis and X-ray Crystallographic Characterization." Polyhedron, 2000, 20, 2637-2646 .
-
Goswami, N.; Eichhorn, D. M.; "Incorporation of Thiolate Donation Using 2,2'-Dithiodibenzaldehyde: Synthesis of Ni, Fe, and Cu Complexes. Crystal Structures of [M(tsalen)] (tsalen = N,N'-Ethylenebis(thiosalicylidene)imine); M = Cu, Ni) Showing a Tetrahedral Distortion of Cu(tsalen)"Inorg. Chim. Acta , 2000, 303, 272-277.
-
Goswami, N.; Eichhorn, D.M.; "A New Method for Incorporating Thiolate Donors into a Metal Coordination Sphere. Synthesis and Crystal Structures of the First Nickel Complexes of an N3S Ligand" Inorg. Chem. 1999, 36, 4329-4333.

